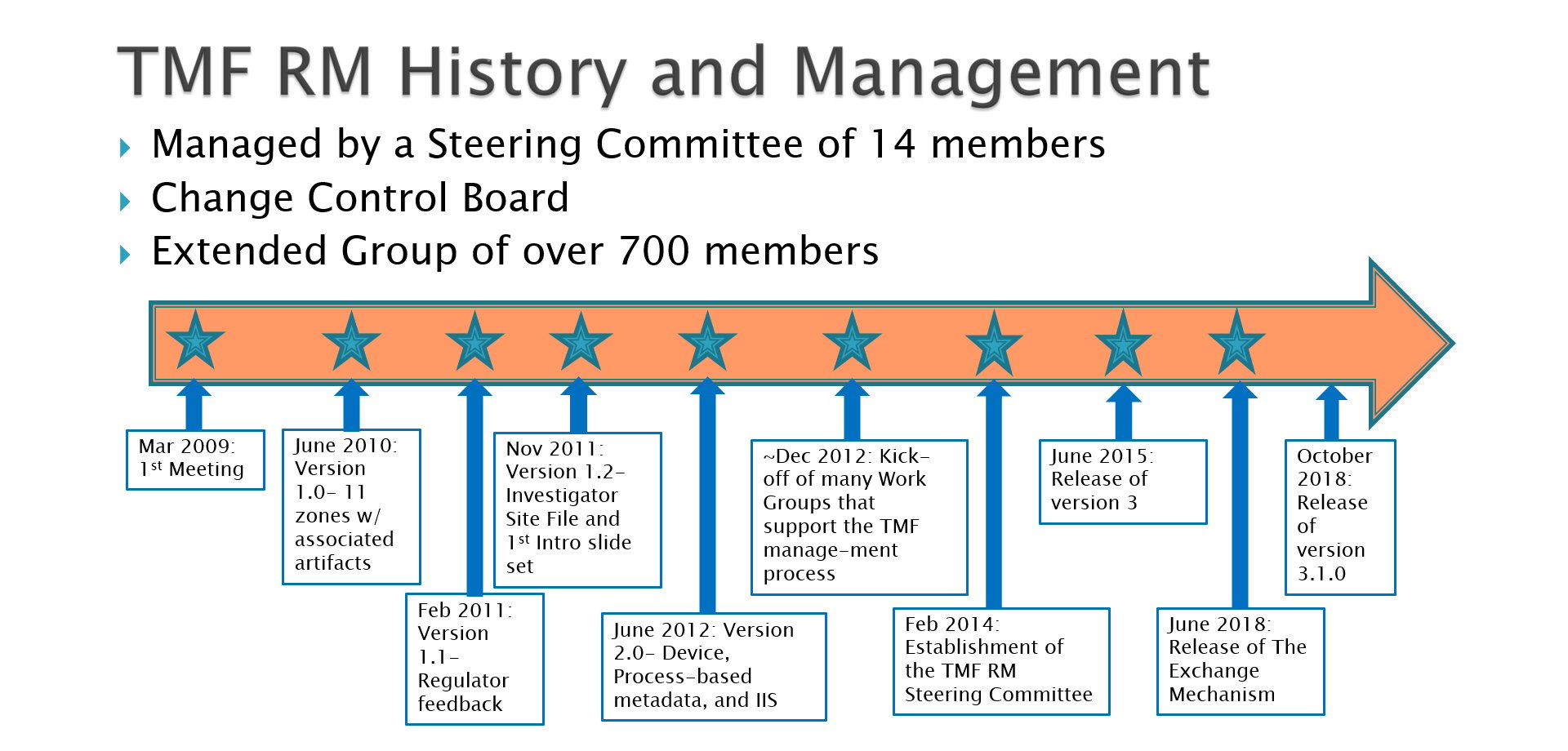
Almost exactly 10 years ago – on the 23rd of March, 2009 to be precise – a pretty diverse group of about 20 TMF practitioners, regulatory professionals, clinical trial managers, document managers, quality assurance (QA) people, and others involved in clinical research got together on a global phone call. The objective of the call? To begin developing the framework for what would become the Drug Information Association (DIA) Trial Master File Reference Model.
What sparked that first meeting is an interesting story. Lisa Mulcahy and I had been asked to incorporate a TMF structure into the DIA Electronic Document Management (EDM) Reference Model. The EDM group covered the regulatory area, and were looking to standardize document naming and content around electronic common technical document (eCTD) specifications for the clinical trial dossier. Their assumption was that this would be a relatively minor and straightforward task.
Lisa and I got together and quickly realized that it was actually a much bigger job than the EDM group had thought – and it grew from there. Everywhere we went, we talked about the need for standardization around the TMF, and worked to recruit people to the group.
And in fact recruiting people was perhaps the easiest part. We had 20 people from multiple areas and from all over the world in that first meeting and all of us recognized that this was a critical problem that needed to be solved.
The reason is that back then, every single company had its own TMF structure. Nothing was aligned. Organizations had different names for the artifacts and documents, they had documents in completely different orders, and each company had a unique mix of what was included in the Trial Master File and what wasn’t. Some TMFs were limited to clinical operational documents and nothing else. And others covered the whole span of the Trial Master File.
Our goal was to get everybody onto a common structure so that inspectors, CROs working with sponsors, sponsors evaluating potential mergers and acquisitions, and so on could all talk the same language.
During that first meeting we worked out how we were going to divide the Trial Master File up. We came up with the concept of splitting the TMF components into zones, and allocate different zones to people to work on. Everyone went off and many sent in their TMF structures to start. We asked participants to pull together all the artifacts that they thought were in their respective zones. And then we spent hours and hours on planes, trains, and automobiles, working to bring it all together because there was a tremendous amount of overlap.
And from there it was just a lot of meetings and a lot of hard work from all of us to get to v1.0 of the TMF Reference Model. There were weekly meetings of all the zones, and the entire group got together on a regular basis.
I remember one meeting during the European EDM Conference in particular where we shut everyone in a room at six pm – right after the conference had finished. There were about 20 of us, and we all worked into the evening for five hours straight. It was very hard and challenging work, but everyone was dedicated to it. We knew this effort was going to make things much more efficient and accurate, and would improve overall quality and completeness of the TMF. It was all worth it when just over a year after that first meeting, we were able to release v1.0 of the TMF Reference Model at the 2010 DIA Annual Meeting in Washington, DC.
We received a lot of great feedback to that version that helped us improve the model. At one point Ivan Walrath, Eldin Rammell, and I sat down with Andy Fisher from the MHRA to get his thoughts and suggestions on how we could make it better from an inspector’s point of view. Following that, we met with Cynthia Kleppinger and a number of FDA inspectors to get their comments. The result was that in February of the following year – 2011 – we were able to release v1.1 of the TMF Reference Model which included the regulatory input we had received.
Despite all these efforts it took a while to persuade the industry to move toward adopting the model; many people initially just used the TMF Reference Model as a reference to what they already had in place. Frankly, I think everyone was a bit skeptical that it was actually going to be accepted by the industry as it meant a big change to their processes and filing structures. As we got more and more people involved, however, it gained momentum and now you don't see an RFP without a requirement that the eTMF system have the TMF Reference Model as the backbone. And practically no one is implementing an eTMF system without using the TMF Reference Model as its structure.
With steady releases of versions 1.2, 2.0, 3.0 and now the latest version, 3.1, of the TMF Reference Model, more and more organizations – sponsors, CROs, and eTMF technology companies – no longer are simply referencing or even adapting the model, but are now adopting the model outright. Each new version has incorporated improvements that go along with the establishment of a tool that is broadly utilized across clinical R&D companies. Artifacts have been added, removed, or moved, definitions improved, and terminology standardized. The timeline accompanying this article shows at a high level the steady cadence of the TMF Reference Model releases.
The dedicated professionals who work to maintain and improve the TMF Reference Model have also collaborated on many significant reference tools that support the TMF management process. These tools include the TMF Plan, Metrics, Dating Conventions, Inspection Readiness, and much more.
Most recently there was the release of the TMF Reference Model Exchange Mechanism, a set of technical specifications for transferring TMF data between different eTMF systems. These specifications are the first step towards developing an Exchange Mechanism standard. Our goal is to help alleviate the efficiency, accuracy, and quality problems that can crop up when more than one eTMF system is involved in a trial or used between and trial sponsor and their CRO partners.
Looking back over the past 10 years, I think all of us involved in the TMF Reference Model project have a great deal to be proud of. It's an amazing thing to bring an industry together like this; the group even received an award (pictured) from the DIA in recognition of our hard work and accomplishments. We essentially created a Trial Master File industry, where before really it was just a bunch of people working on TMFs in different companies using disparate filing indexes.
And while it’s fun to look back, the future for the model is really exciting. In addition to the benefits that the Exchange Mechanism will bring, we’re in discussions with the International Council for Harmonisation (ICH) about incorporating the TMF Reference Model into ICH Good Clinical Practice (GCP). There’s also a need to get the investigator TMF to be the same as the sponsor TMF. Lots of work ahead – but with the dedication and involvement of so many great industry professionals – currently over 700 people! - I feel confident the next 10 years will bring even more exciting accomplishments.
.png?width=300&name=PharmaLex_RGB%20(1).png)

