Adapted from an April 2019 Phlexglobal webinar presented by Eldin Rammell. For a more detailed discussion of the new EMA guidance around Trial Master Files, the webinar is available on-demand here.
The new European Medicines Agency (EMA) guidance on Trial Master Files – “Guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic)” –was published on 6 December 2018. With the guidance in effect as of June 6, 2019, there is much you should know about its impact on TMF processes, technology, and best practices.
I’ve gone through the guidance carefully, and based on my years of experience with regulatory inspections I’ve pulled out the items that will likely require changes to how you are managing Trial Master Files today.
The good news is that the new EMA guidance forms probably the most detailed guide to TMF best practices out there, and in fact is becoming a de facto industry standard. We obviously have other great regulatory frameworks for clinical trial conduct, from International Council for Harmonisation Good Clinical Practice (ICH GCP) to the Medicines and Healthcare products Regulatory Agency (MHRA) guides. The EMA guidance, however, presents practical guidance specifically for the Trial Master File.
10 Key Takeaways from the New EMA TMF Guidance
The following are key areas you should be actively assessing for their likely impact on your organization:
1) Hybrid TMFs are common and are permitted
Right from the start, the guidance recognizes that there are organizations that are still using paper, there are those who have moved to electronic, and there are those with a foot in both worlds. The result is that the regulations don't mandate any particular format for the Trial Master File.
You must, however, have a clear understanding of why you have a hybrid TMF, and evaluate the pros and cons of different file formats. If questioned by the authorities, you will need to be able to articulate the strategy and rationale behind that choice.
2) There is only one Trial Master File for a study
Another area that the guidance sheds more light on is the nature of the TMF; it notes specifically that there is only one Trial Master File. There can be a sponsor component and an investigator component, but it’s all just one TMF.
Note that this does not mean one system – multiple systems are permitted for holding TMF content. The EMA guidance uses the term, “primary TMF system.” The expectation is that within your organization, there must be one central system (which can be paper or electronic) that holds the majority of the Trial Master File and is clearly identifiable as the “primary TMF.”
As a result, we need to start recognizing the TMF as one “package,” rather than thinking primarily around the sponsor TMF with some investigator records out there as well. Terminology can be confusing since it's not standard: some parts of the world talk about investigator site files, others refer to a regulatory binder, and so on. They are all, however, part of the TMF.
And when you consider Good Clinical Practice (GCP), the ICH notes that the TMF is not simply a file repository. It truly is a trial management system, and having most of your documents within that system facilitates trial management and helps ensure compliance.
3) We receive helpful clarification on essential documents
The new EMA guidance provides useful clarification around essential documents, and actually addresses record types in addition to documents. These include databases as well as unstructured electronic records such as email. And it's not just the primary record itself, but includes things like the audit trails and within computerized systems that includes system logs. So those are all considered essential documents and part of the Trial Master File.
Note, however, that the guidance specifically states that the ICH E6(R2) Chapter 8 list of essential documents “should not be used as a definitive checklist for TMF content. It is not an exhaustive list.” [emphasis mine]
It goes on to note that:
- The TMF should include “any documentation that facilitates reconstructing and evaluating the trial conduct, as part of the TMF” e.g.
- completed forms, checklists and reports, generated from following quality system procedures;
- assay method validation report for analysis of IMP or metabolite(s) in clinical samples;
- documentation to demonstrate validation of trial-specific builds of computer systems
Thus it includes not just the core documents themselves, but any supporting document that shows you followed your quality system. Your best approach is to utilize the TMF Reference Model for building your TMF table of contents – it offers a great framework for building your list of essential documents such that it meets regulatory requirements.
4) Insight into sponsor responsibilities for TMF management of outsourced studies
The new guidance reinforces the fact that from a regulatory perspective, the responsibility for an outsourced Trial Master File lies with the sponsor.
As part of management of a study, your CRO will be generating documentation that should reside in the TMF. What’s more, the EMA guidance notes that the sponsor will need to provide CROs access to sponsor essential documents of the TMF that are “required by the CRO to execute their delegated duties and functions.”
It also clearly states that when it comes to the Trial Master File, the contract in place with your CRO for a study – along with other key documents and procedures (such as SOPS and KPIs) that have been agreed upon – should outline the arrangements for the TMF in some detail.
The guidance provides specific examples of the TMF arrangements that should be documented when outsourcing a study, including:
- which party holds the TMF (or which party holds which parts of the TMF when this is divided)
- the structure and indexing of the TMF
- the access arrangements for the involved parties
- when an eTMF is being used, the details of the system and change control management
- lists of applicable procedures to be followed and training requirements
- type of documents that each party should retain
- arrangements for managing correspondence
- how the TMF would be made available to the competent authorities
- arrangements for oversight of the TMF performed by the sponsor and how this would be achieved (e.g. audit reports and/or monitoring)
The above represents just a subset of the items listed in the new guidance. With the outsourcing of clinical trials increasing in scope, this area presents one of the greatest chances for sponsors to get tripped up during an inspection.
5) We get detailed guidance on technology requirements for Trial Master File management
In today’s world most companies are managing their records electronically, or at the very least have many essential trial documents (see points #3 and #4) in electronic form. Section four of the new EMA TMF guidance offers helpful details around what regulators expect from computerized systems.
According to the guidance, electronic TMFs should enable appropriate security and reliability, ensuring that no loss, alteration or corruption of data and documents occur. The primary eTMF is a system for managing documents that should contain the controls listed below:
- user accounts
- secure passwords for users
- a system in place locking/protecting individual documents or the entire eTMF (e.g. at time of archiving) to prevent changes to documents
- regular backup
- periodic test retrieval or restores to confirm the on-going availability and integrity of the data
- an audit trail in place to identify date/time/user details for creation and/or uploading deletion of and changes to a document (explanation of the deletion or modification, if necessary)
- role-based permissions for activities being undertaken, such as restricted access to files/documents (e.g. randomisation codes and unblinded adverse event data)
- the suitability of the system for archiving purposes should be appropriate
What’s more, the guidance goes on to note that the above principles should also apply to any electronic system defined as part of the TMF, such as an SOP management system or central email repository. In addition, it states that an eTMF system should be validated to demonstrate that the functionality is fit for purpose, with formal procedures in place to manage this process.
6) The investigator components of the Trial Master File are the responsibility of the investigator
In this, the EMA is 100% in accordance with ICH Good Clinical Practice in terms of the investigator trial master file and the responsibilities of the investigator. Although the sponsor can get involved in discussions with investigators about their responsibilities for maintaining site records, you can't lose sight of the fact that the investigator TMF is the responsibility of the investigator – and that they must maintain full control of those records at all times.
7) Investigator control over the TMF extends to archiving
The investigator components of the TMF should still be under the control of the investigator or institution upon archival, and completely independent from the sponsor. The guidance provides some helpful tips. For example, the sponsor can certainly provide financial support to the investigator for archiving, since it can be quite challenging for the site to manage their part of the Trial Master File for the periods of time that we're talking about. Whatever system of archiving is put into place, however, the sponsor should never be in a position to have control over those records.
If you look at the wording within the guidance, the recommendation is to discuss the issue of archiving right at the outset of the trial (see next point). So before site initiation, when you're discussing all other aspects of the clinical trial, you should be talking to the investigator site about their responsibility for maintaining their part of the TMF for the time required.
8) Archiving should be included in your TMF planning from the very beginning
Essentially, whatever system you put in place for archiving has to be seen by the regulators as being suitable for archival purposes. There's a real difference between managing and maintaining a Trial Master File while the study is being conducted, and then managing those documents for a long period of time after the study's closed - sometimes in excess of 25 years.
A key takeaway is you should evaluate how to ensure that the data and the metadata of the TMF is retrievable. The EMA uses the term, "as a usable data set." Think about the structure of the technology that you're using for archiving – whether the TMF is electronic, paper, or a hybrid – and assess whether that technology meets the criteria of “usable data set” for the long term.
Once the study has been archived, you’re also required to conduct periodic quality assurance (QA) activity to ensure that the provider is performing in accordance with the agreed-upon contract, your service-level agreements (SLAs), and so on.
9) Avoid unnecessary duplication
I think it's interesting that the EMA guidance doesn't say “avoid duplication” – it says to avoid “unnecessary duplication” (emphasis mine). So there might be occasions where it makes sense to duplicate items, but where you can avoid it, do so. For example, the guidance specifically notes that “documents applicable to multiple trials do not need to be duplicated in several TMFs.”
There are a couple of examples that come up frequently. I'm often asked about clinical trial approval documents, which as you know will typically have a cover letter or a form, accompanied by documents like the protocol, a blank CRF, and a diary card. Do all these have to be filed with the Clinical Trial Agreement (CTA)? If those documents already exist within your eTMF system, then that may be unnecessary duplication. A better approach would be to provide a pointer to those documents.
10) Certified copies create lots of confusion (the guidance helps)
I think I've fielded more questions about certified copies over the last year than any other topic. In a future blog, I’ll cover this in more detail, but following are a few key points from the guidance.
The good news is that the new EMA TMF guidance is fully aligned with FDA as well as ICH regulations and guidance, and provides excellent guidelines based on document management best practices. If you adopt these guidelines, you will not only be compliant but as a result also improve the quality of your Trial Master File in areas of completeness, accuracy, and readability.
More good news: not every copy in the TMF has to be a certified copy. Certified copies are only mandatory when the copy that you have in the TMF irreversibly replaces an original. So if the original is destroyed, either accidentally or deliberately, then the copy that is in the TMF must be a certified copy to be acceptable.
The new EMA TMF guidance allows for two methods of producing a copy (see Figure 1). Whatever method you choose is fine, but then you need to be consistent with that approach within your organization.
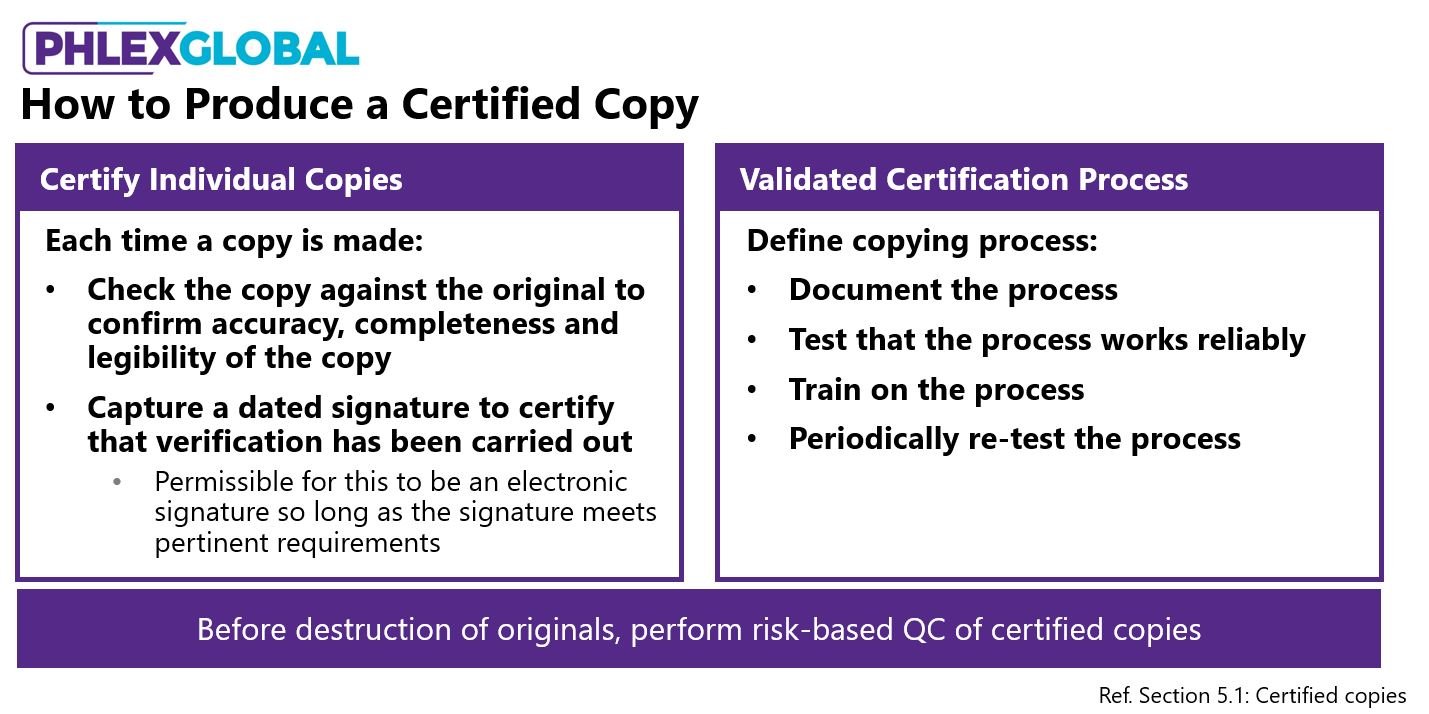
Figure 1. The EMA guidance provides for two approved methods to generate certified copies – but you must choose and consistently apply only one of these approaches.
I’ve really just scratched the surface of the potential impact of the new EMA TMF guidance on your organization’s Trial Master File and related practices. There are other areas to be aware of – such as the management of email – which we covered in our recent webinar. I also encourage you to review the guidance itself, available here.
Looking for more information? Download the full guide here.
Phlexglobal is offering this free guide by renowned TMF expert Eldin Rammell to help you better understand the impact this can have on your TMF management processes.
Get your copy here.
.png?width=300&name=PharmaLex_RGB%20(1).png)


