Adapted from a Phlexglobal Ask An Expert interactive discussion, “How to Ensure TMF Inspection Readiness & Compliance for a Recently Acquired Product,” held March 2, 2022 and available on-demand here. Third in a series; you can read the first blog - “Why Is the Trial Master File Critical in M&A Activities?” – here and the second blog – “Congratulations! You Just Acquired a Trial Master File. Now What?” – here.
As mentioned before in this blog series, an acquired Trial Master File (TMF) requires special attention to ensure that essential content is available, documented, and organized appropriately. A deeper analysis of the TMF and its components is likely necessary to ensure inspection-readiness. However, the analysis should provide tactical and strategic insights so your team can reflect on it, take action, and communicate the strategies and remediation plans to management and other functional areas within your organization.
Phlexglobal recommends two expert methodologies and tools that help TMF owners establish the current state of the TMF, and better understand what steps are needed to maintain compliance: TMF Quality Review and TMF Heatmaps.
TMF Quality Review: Expert Insight Improves Inspection-Readiness
A TMF Quality Review is a comprehensive evaluation providing critical details on the quality, completeness, and timeliness of the Trial Master File. It includes a complete inventory of all content, line-by-line details for all required actions, and a comprehensive gap analysis identifying missing documentation.
An effective TMF Quality Review should:
- Be conducted by experienced TMF practitioners who can bring deep understanding of current inspection standards at health authorities
- Include documents and data from ANY relevant source, whether paper, shared online folders, disk drives, a dedicated eTMF system – or some combination of these
- Incorporate risk-based assessments to quickly pinpoint and drill down to problem areas
- Provide meaningful and pragmatic insights, together with detailed, expert recommendations for any necessary remediation
If required, an expert TMF Quality Review should also have the flexibility to be conducted on a wider scale across multiple studies, to provide broader, portfolio-level visibility into the health and current state of TMF assets.
TMF Heatmaps: A Visual Guide to TMF Health
TMF Heatmaps offer a snapshot view of where documents may be missing in the study. They contain visual and textual overviews of TMF findings and help identify recurring patterns as well as major and minor areas that should be addressed. Heatmaps are valuable tools for managing and communicating the rationale and priorities for remediation strategies. For example, if there were patients in one country where entire sections of the TMF are missing, the visual insights help teams quickly identify the issue and initiate conversations to track down missing documents and data.
Heatmaps offer practical insights regarding the actions necessary to improve TMF health and inspection-readiness. For example, logic checks and cross-checks validate the quality of individual documents and whether documents all relate to each other properly.
|
|
|
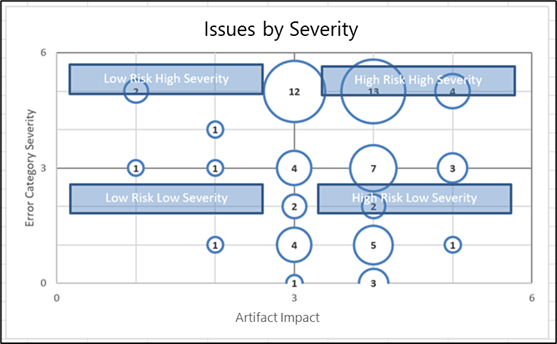
To be effective, a TMF Quality Review should include visualizations such as heatmaps to guide risk mitigation. This includes root cause analysis as well as recommended corrective actions such as TMF structure refinement, clarification of document expectations, or SOP changes. For example, by mapping Artifact Impact Level against Error Category Severity, you can prioritize efforts around the highest risk and impact. |
TMF Views and Insights
TMF Quality Review and TMF Heatmaps offer unprecedented transparency into the overall health of the Trial Master File, while offering a roadmap to improve TMF compliance. These advanced tools have been rigorously used and proven to turn poor-quality Trial Master Files into inspection-ready assets.
--------------------------------------------------------------
Need help or have questions about an acquired a Trial Master File? Request a complimentary assessment from one of our TMF experts today.
Want more on this topic?
.png?width=300&name=PharmaLex_RGB%20(1).png)