Migrating Trial Master File (TMF) data is a fairly common occurrence, usually driven by one or more of the following scenarios:
- Implementing a new electronic Trial Master File (eTMF) system
- Transferring a TMF from a Contract Research Organization (CRO) to the trial sponsor
- Conducting a TMF transfer from one CRO to a different CRO
- A trial sponsor has acquired or divested an asset
- Corporate mergers and acquisitions
- Consolidating eTMF info (e.g. from Safety / Regulatory systems, or EDC, into a core eTMF)
Performing a TMF data migration, however, can be fraught with risk. In a recent Phlexglobal “Ask An Expert” webinar (available on-demand here), we polled our audience of 119 industry professionals regarding their most common TMF migration challenges. The results of that survey (see Chart 1) aligned with Phlexglobal’s own experience conducting hundreds of successful TMF migrations, with corrupt and/or missing data a recurring problem that we often see as well.
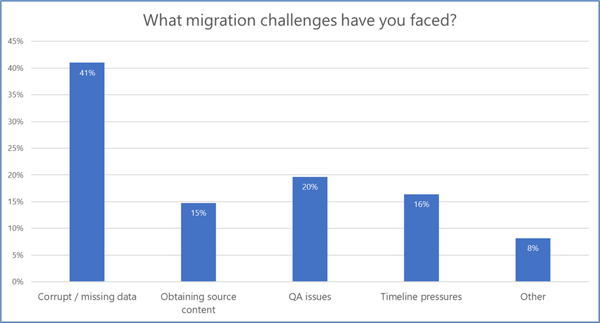 Chart 1. Results from a poll of 119 attendees of the Phlexglobal Ask An Expert webinar, “TMF Migrations: Streamlining the Journey to an Inspection-Ready Result,” held September 12, 2023. Watch the webinar.
Chart 1. Results from a poll of 119 attendees of the Phlexglobal Ask An Expert webinar, “TMF Migrations: Streamlining the Journey to an Inspection-Ready Result,” held September 12, 2023. Watch the webinar.
In this blog, we summarize some of Phlexglobal’s key tips, checklists, and lessons learned that can help reduce the risk and effort involved in migrating a Trial Master File. Refer to the above-mentioned webinar for a deeper dive into the topic, read our step-by-step guide to TMF migrations, or contact us with any questions – our TMF experts are happy to help.
Why You MUST Have a Migration Plan
Figure 1 shows the overall flow of a TMF migrations project, based on Phlexglobal’s extensive experience successfully managing hundreds of TMF migrations. For the purposes of this blog, we focus on the key element that can mean the success or failure of your migration: upfront planning.
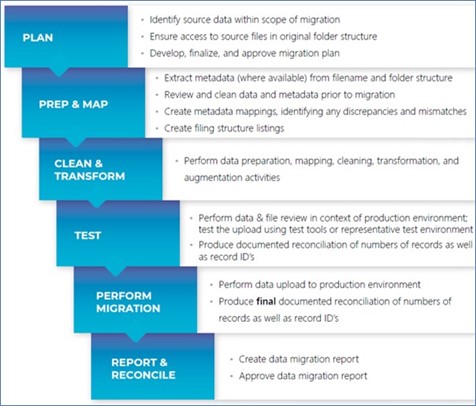 Figure 1. An overview of Phlexglobal’s process for managing TMF migrations, built upon successfully migrating hundreds of TMFs.
Figure 1. An overview of Phlexglobal’s process for managing TMF migrations, built upon successfully migrating hundreds of TMFs.
Every successful TMF migration starts with an effective plan. What’s more, everyone involved in the migration needs to review and sign up to the plan before you begin.
A good place to start is capturing the overall project scope before drilling down into the many details (see Figure 2).
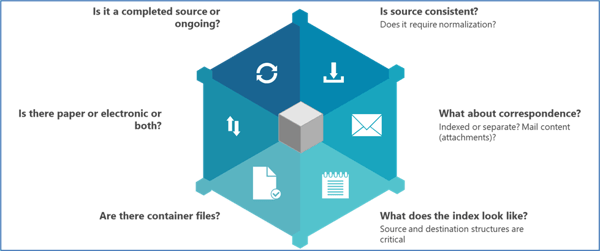 Figure 2. An example of a high-level approach to scoping a TMF migration project when building a migration plan, based on Phlexglobal’s Migration Standard Operating Procedures (SOPs).
Figure 2. An example of a high-level approach to scoping a TMF migration project when building a migration plan, based on Phlexglobal’s Migration Standard Operating Procedures (SOPs).
Why are these important? To take just two examples:
Is the study (source) completed or ongoing?
Migrating live studies brings its own unique set of complexities. We sometimes find organizations do not have a clear project plan that was communicated to and followed by all personnel, with the result that data has changed after the migration expert. The result? All the work has to be redone from the beginning (together with any plan updates and additional training required to avoid the same issue from recurring).
Are there container files?
What we call “container” formats are becoming much more common, and create significant challenges when migrating a Trial Master File. These are especially difficult since they can contain multiple layers of documents, data, and correspondence. Think of a PDF compiled from multiple source formats, or files in Outlook Item (.msg) format, which can encompass numerous individual email messages (many with their own attachments), tasks, and so on. These container files are a primary cause of duplicated documents in the migrated TMF, since it can be extremely difficult to parse all the documents for comparison with other sources.
Once you have the project’s big picture, there are then a host of critical details to consider that organizations often overlook. The following questions can serve as a good checklist to help avoid many of these:
- Do we understand the source system versioning logic?
- Do we understand the audit log formats and requirements (since every eTMF source system is different)?
- Is there a special consideration to signed or approved content (e.g. electronic vs. digitally signed)?
- Do we have a process to ensure there is no duplicate content?
- How do we incorporate renditions (exports) generated by eTMF systems? Recreate, reuse?
- Is a production transition required (e.g. black-out periods for system/users for live studies)?
- What are all the user access/permissions considerations involved?
- Is secondary metadata available? Is there a place for it in the destination system?
- Should the final data be superseded in or deleted from the source?
What’s Below the Surface?
As noted earlier, this blog represents just the tip of the iceberg that is a Trial Master File migration project. At Phlexglobal, our experience conducting hundreds of TMF migrations, including all manner of source and destination environments, has allowed us to develop a set of core best practices and Standard Operating Procedures (SOPs) that are the key to a successful migration.
Want to help ensure an inspection-ready outcome for a TMF migration? Request a TMF migration assessment.
Like to learn more? Visit https://www.phlexglobal.com/migrations-and-imports
Disclaimer
The contents of this article contain marketing statements from Phlexglobal Ltd., a subsidiary of Cencora, Inc. Phlexglobal and Cencora strongly encourage readers to review all available information and to rely on their own experience and expertise in making decisions related to the content contained herein.
.png?width=300&name=PharmaLex_RGB%20(1).png)

