You’re responsible for preparing your Trial Master File for an upcoming inspection or audit. You’ve inherited some TMFs in bad shape, from missing essential documents to problems accessing critical files; the study teams don’t seem to be filing as much this quarter; you just discovered a CRO does their quality check at the same they submit the document … with the same person doing both. How are you going to ever going to get your TMF inspection-ready?
To do inspection-readiness well and make it stick, you need a plan that goes beyond learning about the regulations and writing new procedures, more than developing metrics and creating dashboards, more than creating another online learning module. You need to consider human behavior and how we fail to develop habits that take care of our future selves, or in this case, our TMF deliverables.
We’ll start with the common behavioral obstacles to achieving and maintaining good quality habits.
The Magic Bullet Fallacy
Have you ever been to dinner where everyone solves the world’s problems over a glass of wine and crème brûlée? Too often, the same approach is used to improve TMF within complex organizations. Decisions are made, someone is tasked with writing a new procedure or measuring a quality threshold or developing an information campaign and … nothing changes.
The above scenarios are the Magic Bullet Fallacy at work. We take an action and believe that by making the decision, it controls the outcome. Organizations need to do more than provide training on new procedures and measure training compliance. Dan Parisi, Executive Vice President at management consulting firm BTS, has noted that “We repeatedly see one-way communications being delivered by senior leaders during town hall meetings, off-sites and more. The audience passively receives the one-way transmission and is expected to go demonstrate new behaviors to support execution.” (BTS Special Edition Journal, December 2016). We need to do more. We need to measure proficiency in adopting new quality habits.
The Last Mile (or Kilometer) Is the Hardest One
In addition, we need to address what is commonly referred to as the Last Mile Problem. While this challenge usually refers to the logistics and delivery of goods, it equally applies to the delivery of information. Often there is an assumption that information flows like water in a smooth, steady stream from Leadership to Management to Front Line Staff, but information (along with misinformation and disinformation) flows through every organization more like a mountain river – some water gushes rapidly to its destination; some stops and hides in shallow pools, later rejoining the flow; some branches off into tributaries, ending up in a completely unforeseen destination. Not unlike the results of the Telephone Game, the received information (when it is received) is often a distortion or degradation of the original message.
In the world of clinical development, this means that what everyone involved in a study – from the head of clinical operations and study managers, to document managers and CROs – understand to be the “truth” can be very different, even among members of the same team.
False Urgency Makes Achieving TMF Health More Difficult
When the magic bullets have failed to hit their targets and the information gap of the last-mile problem raises its head, False Urgency emerges to create yet another challenge to ensuring Trial Master File quality and inspection-readiness when managing documents. Constant email interruptions to resubmit a document or continually logging into the eTMF to fix metadata degrade the entire quality process.
Francesca Gino and Bradley Staats describe this problem in the Harvard Business Review (March 22, 2016). “People routinely feel pulled between tasks that demand immediate attention and tasks that are important, the ones that bring them closer to achieving their long-term goals. Unfortunately, our and others’ research shows that people have a natural tendency to overly focus on the former (such as responding to mundane emails) at the expense of the latter.”
“What Can I Get Done Today?” vs. “What Should I Get Done Today”
A final behavior is worth noting that ultimately degrades the quality of your TMF (and you know it well). It’s called Zipf's Principle of Least Effort, and refers to choosing the path of least resistance. At work, where workloads often exceed the available hours in the day, everyone at every level of your organization is calculating risks associated with their workloads and using the least effort to accomplish what they identify as most important (or most important to their bosses … which is often not the quality of the TMF).
Applying the Power of Habit to Improving TMF Quality
The way to address the above common human behaviors that interfere with quality in a regulated industry is to establish Quality Habits across your organization and at all levels. Habits counteract the exceptions (“I’ll file that document later”; “I’m sure that will get covered in QC”; etc.) you make today … and tomorrow … and over and over until they become patterns not exceptions.
Developing healthy habits is how we get ourselves to the gym today versus a promised tomorrow that never comes. Developing habits at an organizational level utilizes the same feedback loop that works on an individual level. In his 2012 book The Power of Habit, Charles Duhigg explains the three facets of a habit loop: the cue or trigger, the routine, and the reward.
Here is just one example of this process applied to a Document Submitter.
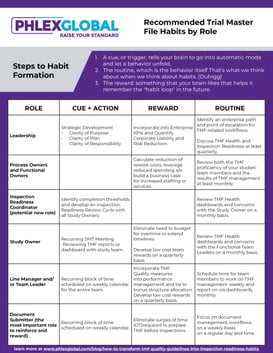 Step 1: Create a recurring cue on your weekly schedule to focus on building the TMF, preferably at the quietest time on your schedule.
Step 1: Create a recurring cue on your weekly schedule to focus on building the TMF, preferably at the quietest time on your schedule.
Step 2: Develop your routine based on the cue. At the scheduled time, log out of your email, and focus on submitting documents or responding to quality issues - your routine. By associating Trial Master File management with a regular time of the day and week, you will begin to complete this important work with less and less effort. The beauty of routines involves reducing mental resistance to accomplishing tasks that feel unpleasant. Thursday afternoons after lunch, or Friday mornings before opening email, will become “TMF Time.”
Step 3: Create your reward for your accomplishing your routine. While one reward for this recurring behavior is clearly improving the quality of the TMF at a study and organization level, human beings need personal and meaningful rewards to fully establish a habit loop. Give yourself a break outside when you have completed your time working on the TMF, and follow up this time doing the work you enjoy the most. Those “urgent” emails can wait – unless that genuinely gives you pleasure. Find a reward that works for you.
CLICK HERE TO DOWNLOAD THE "RECOMMENDED TMF HABITS BY ROLE" INFOGRAPHIC.
As a global clinical research community seeking to improve the health and well-being of patients, we have developed Good Clinical Practice (GCP) guidelines to counter the human behaviors that have often led to poor (if not atrocious) patient safety and questionable data integrity. We develop regulations, pass laws, write Standard Operating Procedures (SOPs), and measure training compliance to address the fallibility of human behavior in our research. Focusing on the development of quality TMF habits will fully address our behavioral limits resulting in poorly managed – and low-quality – Trial Master Files.
Ask your team: What do they consider cues, routines, and rewards to improve the management of your TMF? You’ll find the path to transforming Trial Master File quality guidelines into effortless, everyday habits will be easier than you think.
To learn more about developing and implementing better habits to improve TMF quality, watch the on-demand webinar presented by Marc Webb.
.png?width=300&name=PharmaLex_RGB%20(1).png)

