At the 2022 Drug Information Association Annual Meeting, held in-person in Chicago June 20-23, representatives from the compliance offices of the FDA and MHRA gave a detailed assessment of the pros and cons of remote inspections for Good Clinical Practice (GCP). Driven by the COVID-19 pandemic, regulatory agencies and industry have been forced to grapple with how to conduct effective inspections, while maintaining public health protocols.
The key takeaway? While challenging, remote inspections – as well as their hybrid cousins (conducted remotely but with some level of on-location presence by regulators) – played an important role in most regulators’ inspection toolkits (see Figure 1), and will continue to be utilized going forward.
As noted during the session:
“The innovative alternative methods FDA adopted were critical in informing the Agency’s regulatory decisions for marketing applications while mitigating the spread of COVID-19.”
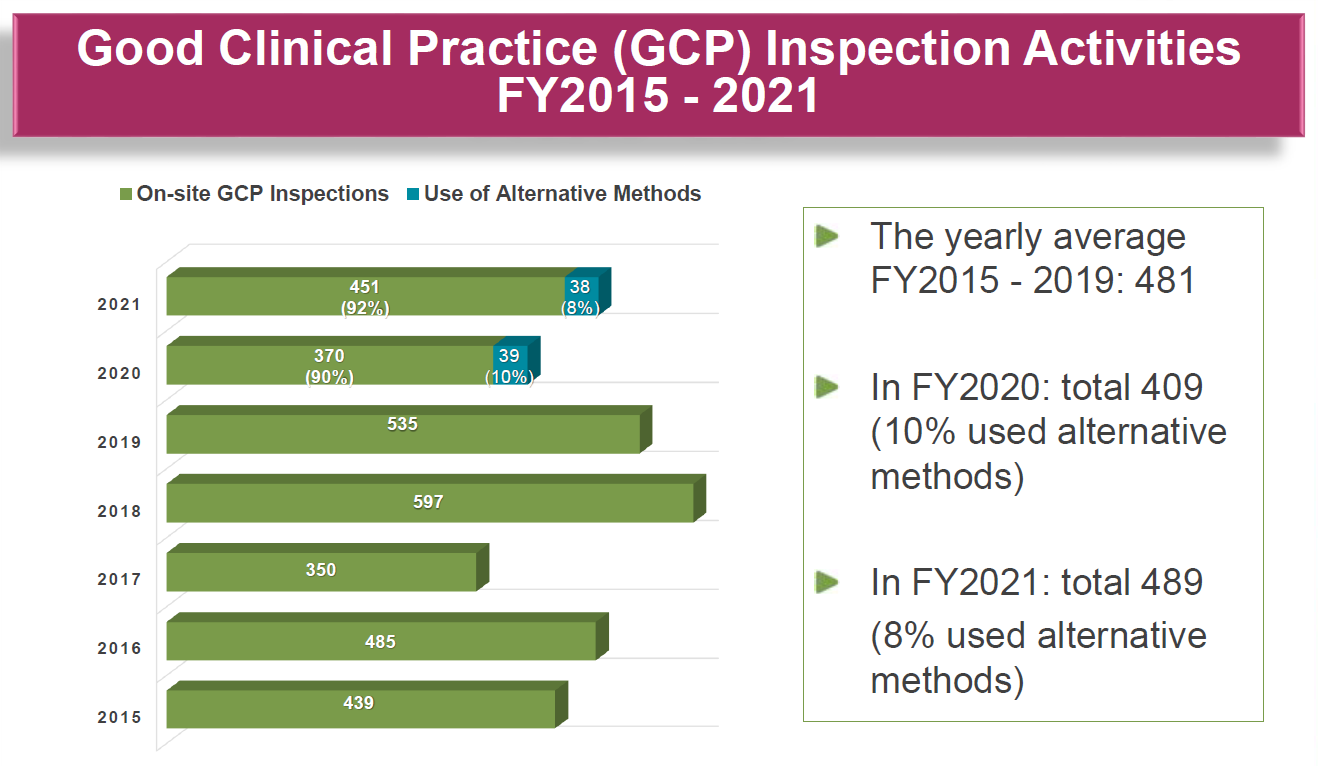
Figure 1. Graphic demonstrating the pandemic-fuelled rise in alternative inspection methods at the FDA.
Following is a brief summary of the session, together with the basis for the MHRA presentation – a reflection paper published in December 2021 by the International Coalition of Medicines Regulatory Authorities (ICMRA). Titled “Reflections on the regulatory experience of remote approaches to GCP and GMP regulatory oversight during the COVID-19 Pandemic,” the paper was developed by an ICMRA working group chaired by the MHRA.
You can access the full paper here: https://www.icmra.info/drupal/sites/default/files/2021-12/remote_inspections_reflection_paper.pdf.
The FDA Perspective on Remote GCP Inspections
For GCP document evaluations, the FDA divides remote inspections into three categories of what it calls “Remote Regulatory Assessments (RRA)”. Figure 2 shows a breakdown of the application of each method.
FDA’s definition of RRAs:
- Remote evaluations of GCP documents at FDA locations
a. A voluntary request to remotely review clinical trial documents at the sites of clinical investigators (CIs), sponsors/contract research organizations (CROs) to ensure data integrity and subject safety.
b. Performed in accordance with Bioresearch Monitoring Program (BIMO) Compliance Programs for CIs, sponsors/CROs.
c. Used technologies allowing for video conference, teleconference, data sharing, and read-only remote access to study records. - Remote evaluations of GCP documents from non-US sites at the U.S. sponsor or agent locations
a. Remote evaluations of non-US CIs conducted at sponsors’ locations in the U.S. due to:
i. Travel restrictions to the non-US CI sites due to pandemic
ii. FDA’s current restrictions on conducting inspections in some parts of the world
iii. Data privacy consideration for CIs in the European Union (EU) member states
b. Remote evaluation of a non-US sponsor at sponsor’s US agent location - Review of GCP documents obtained through FDA’s information request
a. Used for evaluations of non-US CIs when other RRAs were not feasible
b. FDA inspectors reviewed the following documents submitted by the sponsors:
i. electronic case report forms
ii. monitoring reports
iii. lab reports
c. Review was limited to the documents available
d. No interactions with the inspected entities
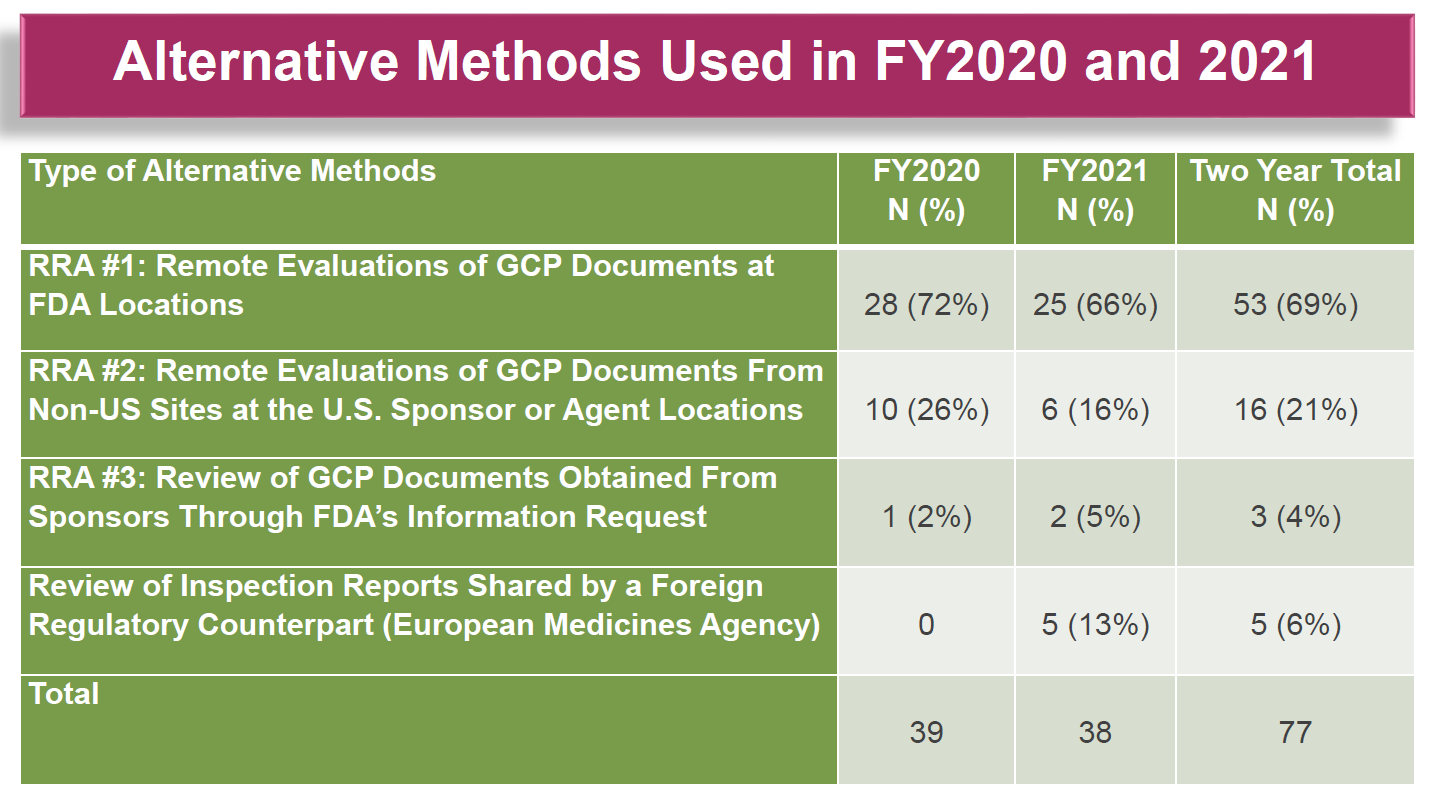
Figure 2. Breakdown of Remote Regulatory Assessments (RRAs) conducted by the FDA during the first two years of the COVID-19 pandemic.
For its remote inspections of sponsors, the FDA’s focus was on a few critical areas:
- Trial Master File
- Transfer of regulatory obligations to contractors
- Data management
- Monitoring
- Study drug handling and accountability
In general, the FDA’s experience with these alternative inspection methods was positive, observing that:
“Remote Regulatory Assessments (RRAs) were useful in evaluating CI and sponsor/CRO data integrity, subject safety, and clinical trial conduct. Looking forward, RRAs can be complimentary to on-site GCP inspections, particularly when travel limitations exist.”
ICMRA Working Group: Remote Inspections Reflection Paper
The remote inspections working group referenced previously was chaired by MHRA, and included representatives from FDA, EMA, Health Canada, Swiss-medic, HPRA Ireland, AEMPS Spain, ANSM France, PEI Germany, MHLW/PMDA Japan, TGA Australia, ANVISA Brazil, HSA Singapore, WHO and Saudi-FDA.
Due to the often ambiguous terminology around “remote inspection,” for its paper the working group developed the core set of terms and definitions shown in Table 1.
|
Term |
Definition |
|
On-site Inspection |
An inspection conducted physically on-site. |
|
Remote inspection / Distant assessment / Evaluation |
The process of conducting inspections, evaluations or assessments at a distance/virtually, supported by technology for communicating, sharing, reviewing, accessing systems, without the inspectors being physically present at the sites where the activities subject to an inspection have taken place/where the inspection would routinely be hosted. |
|
Hybrid Inspection / assessment |
An inspection/assessment performed using a combination of on-site and remote means. |
|
Collaborative inspections |
Inspections involving two or more regulatory authorities. |
Table 1. Terminology developed by the ICMRA remote inspections working group.
Digital technologies involved in remote inspections included electronic trial master files, electronic case report forms, electronic patient reported outcomes, interactive response technologies, and electronic document management systems. The ICMRA working group noted, however, that paper documentation remained a major stumbling block:
“Extensive paper-based source records systems have not been found amenable to remote inspections.”
One digital challenge identified by the ICMRA working group included direct access to documentation: “For GCP inspections, some electronic systems owned and managed by service providers, (CROs) and sponsors, have not been accessible remotely due to their internal business confidentiality rules as these systems contain other organisations’ (sponsors, vendors, third parties) information not related to the trial(s) subject or data to be inspected.”
In addition, the group noted that the IT infrastructure and policies of both the regulator and the organization being inspected had an impact on the success of the inspection. To help mitigate this challenge, the reflection paper suggests getting IT representatives from both parties involved in the early stages of logistical discussions.
Overall, the ICMRA reflection paper found that the use of digital technologies in the remote conduct of inspections, evaluations, and assessments presented a “key business continuity tool for regulatory oversight.” Trial sponsors and their service providers should take note, and be prepared for the full spectrum of remote, on-site, and hybrid inspections.
Have questions about inspection best practices, including how to avoid critical findings? Download our guide, “5 Reasons Your TMF Isn’t Inspection-Ready (and what you can do to fix it).” Or, contact us today to speak with a Phlexglobal TMF inspections expert.
.png?width=300&name=PharmaLex_RGB%20(1).png)

