Companies significantly increasing speed and accuracy of document processing and quality control with PhlexTMF’s AI-assisted indexing solution trained on millions of Trial Master File documents
Amersham, UK; Malvern, PA. — June 2, 2021
Phlexglobal, a leading innovative technology and services provider for the life sciences industry, today announced benchmarked results from the industry’s most advanced electronic Trial Master File (eTMF) solution leveraging advanced artificial intelligence technologies. Pre-trained on millions of documents before being deployed in 2020 to Phlexglobal customers, the groundbreaking AI-assisted indexing functionality within PhlexTMF eTMF software has now processed more than one million real-world Trial Master File documents.
The results to date demonstrate significant benefits for resource-constrained organizations: An average 16% reduction in document processing time into PhlexTMF, and a classification accuracy rate of 95%. For those companies that produce 100,000’s of documents this would result in a significant reduction in staff workload.
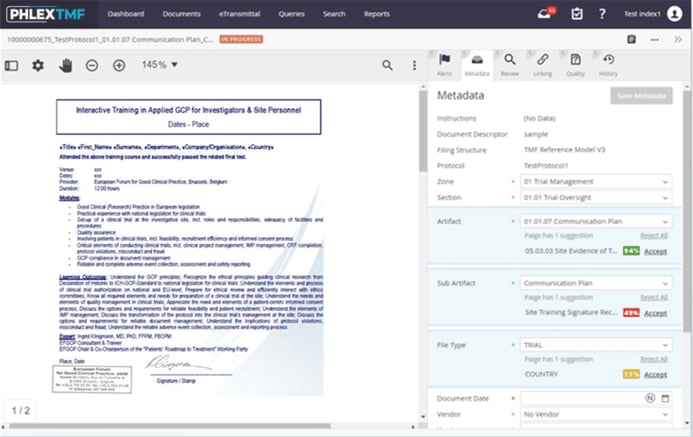 Phlexglobal’s PhlexTMF software improves TMF processing speed and quality through the industry’s most advanced TMF automation capabilities, utilizing machine learning algorithms trained on millions of TMF documents.
Phlexglobal’s PhlexTMF software improves TMF processing speed and quality through the industry’s most advanced TMF automation capabilities, utilizing machine learning algorithms trained on millions of TMF documents.
“Document processing is a use case absolutely tailor-made for artificial intelligence, which is why we’ve selected it as the first of many AI capabilities on our eTMF software roadmap.
Jim Nichols
Chief Product Officer
PhlexTMF automatically classifies TMF documents and presents the confidence level of its classification for human reviewers for each artifact, sub-artifact, and file type
Karen Roy
Chief Strategy Officer
“PhlexTMF automatically classifies TMF documents and presents the confidence level of its classification for human reviewers for each artifact, sub-artifact, and file type,” noted Karen Roy, Phlexglobal’s Chief Strategy Officer. “This can save thousands of work-hours and help enable risk-based QC methods endorsed by regulations. And for those concerned about automation impacting their jobs, our customers are actually shifting study teams from low-value manual effort to higher-value areas, improving overall TMF quality.”
Regulatory agencies have significantly increased their scrutiny of the Trial Master File during inspections, considering the TMF a key performance indicator (KPI) for GCP compliance during a study. As a result, inspectors no longer just look primarily for completeness, but expect a high degree of accuracy as well. They want documents filed in the right place consistently, and to meet the proper quality standards with minimal duplication.
Powered by PhlexNeuron, Phlexglobal's proprietary and sophisticated machine learning framework, PhlexTMF provides automated extraction of specific data from structured and unstructured data sources including documents and emails. With millions of documents already processed, together with ongoing adjustments to the algorithms by Phlexglobal’s pool of more than 150 TMF experts worldwide, the accuracy and efficiency of the PhlexTMF algorithms improve continuously and at an ever-accelerating rate.
“Document processing is a use case absolutely tailor-made for artificial intelligence, which is why we’ve selected it as the first of many AI capabilities on our eTMF software roadmap,” observed Jim Nichols, Phlexglobal’s Chief Product Officer. “Implemented as part of TMF management workflows, such as automatically prioritizing a larger sampling of high-risk or essential artifacts for quality control, means that even these significant initial efficiency and quality gains will quickly be surpassed.”
As just one example, optical character recognition (OCR) and language detection in PhlexTMF logic, combined with PhlexNeuron AI, can identify quality issues with essential documents – such as whether the pages are rotated, or where there's poor scan quality. Instead of a reviewer spending time on these types of simple tasks, the system notifies users whether the text is readable and quality content can be extracted.
“The new AI-powered capabilities provided by PhlexTMF further showcase Phlexglobal’s innovation and leadership in delivering on the promise of digital transformation in life sciences, which has been accelerated by the COVID-19 public health crisis,” said John McNeill, CEO of Phlexglobal. “At the same time, we are committed to the highest data security, integrity, and privacy standards regarding the use of clinical data in our AI applications and indeed across all our software and services. It’s only with complete transparency and ethical dealings among all parties that our industry will truly benefit from ongoing innovation.”
UK: +44 (0) 1494 720420
US: +1 (484) 324-7921
Poland: +48 81 45 46 132
Germany: +49 89 23514741
E-Mail: info@phlexglobal.com